Palladium-Catalyzed Coupling of Ammonia with Aryl Chlorides, Bromides, Iodides, and Sulfonates: A General Method for the Preparation of Primary Arylamines | Journal of the American Chemical Society

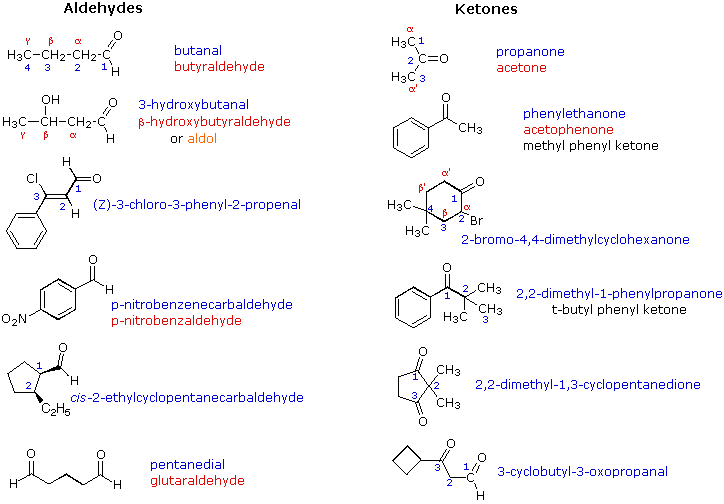
Schematic derivatization reaction of 1-phenyl-2-propanone (A) to oxime... | Download Scientific Diagram

Mechanistic Studies of Palladium-Catalyzed Aminocarbonylation of Aryl Chlorides with Carbon Monoxide and Ammonia. - Abstract - Europe PMC

TPD analysis of the adsorption of ammonia on Pd–I catalyst and MCM-41. | Download Scientific Diagram
Chapter 21: Amines. Organic derivatives of ammonia, NH3. Nitrogen atoms have a lone pair of electrons, making the amine both ba

EP1735266B1 - Process for preparation of optically active 1-erythro-2 -amino-1-phenyl-1-propanol - Google Patents

Probing the effect of donor-fragment substitution in Mor-DalPhos on palladium-catalyzed C–N and C–C cross-coupling reactivity



![What is di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii )?_Chemicalbook What is di-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii )?_Chemicalbook](https://www.chemicalbook.com/NewsImg/2020-2-12/2020212170369170.jpg)

![Large-Scale Methamphetamine Manufacture from P2P - [www.rhodium.ws] Large-Scale Methamphetamine Manufacture from P2P - [www.rhodium.ws]](https://www.designer-drug.com/pte/12.162.180.114/dcd/chemistry/pictures/meth.louisfreeh-18.jpg)
![Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws] Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/pictures/p2p.grignard.gif)

![Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws] Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/pictures/p2p.ylide.gif)
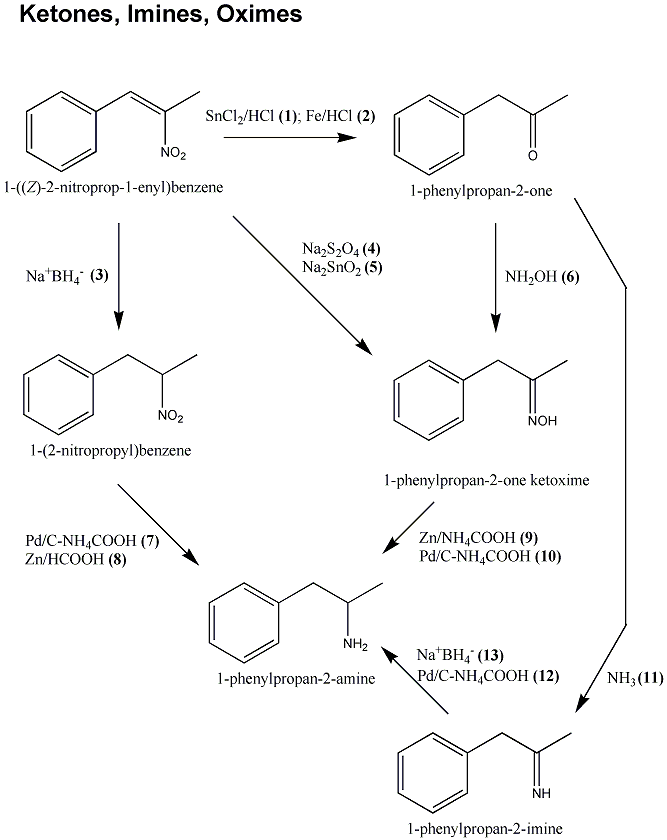
![Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws] Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/pictures/p2p.ephedrine1.gif)
![Synthetic Reductions in Clandestine Amphetamine and Methamphetamine Laboratories - [www.rhodium.ws] Synthetic Reductions in Clandestine Amphetamine and Methamphetamine Laboratories - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/pictures/amph-red-03.gif)
![Large-Scale Methamphetamine Manufacture from P2P - [www.rhodium.ws] Large-Scale Methamphetamine Manufacture from P2P - [www.rhodium.ws]](https://www.designer-drug.com/pte/12.162.180.114/dcd/chemistry/pictures/meth.louisfreeh-15.jpg)